
Creating
the Context
Home
Research Focus
Background Info
Research Methods
Data Submission
Results of Study
Data Analysis
Conclusion
Further Research
Guided
Research
Research Question
Background Info
Research Methods
Data Submission
Results of Study
Data Analysis
Conclusion
Further Research
Research Values
Student
Research
Doing Research
Publish
View
Tools
Discussions
Email List
Lichen Links
Lichen Map
Image Bank
Project Awards
|
|
Sampling Procedure for Lichen Coverage
The following procedures will standardize the lichen coverage measurements so results can be compared and analyzed with other sites.
- Select 10 mature trees for your sample. These ten trees should be of the same species, if possible. Mark the tree for later identification. At each selected mature tree of your selected species, tie a string around the trunk at a height of 1.5
meters from the ground.
- Collect the Latitude and Longitude for each tree in your sample. A USGS topographic map may be used to determine the Latitude and Longitude, or you can borrow a Global Positioning Satellite Unit from the PathFinder Science project. As an alternative, use trees that are within one kilometer (.6 mile) of your school site and record the latitude and longitude of your school site on the data collection table.
- Record the latitude and longitude of each tree on the data collection table.
- Try to choose trees with alkaline bark, preferably ash, but if not ash, then elm or sycamore. If need be, use trees with acid bark, preferably oak, but if not oak, then beech or birch.
- Scrape some bark from the tree into distilled water (pH 7.0) and let it soak. After 24 hours, take a measurement with a pH probe, if you have access to one, or with a pH strip test.
For each tree, fill in the information on the table below.
| School Name |
|
| Sample Date |
|
| Tree Number |
|
| Tree Species |
|
| Latitude |
|
| Longitude |
|
| pH of the Bark |
|
To estimate the degree of cover we will use a belt transect
with accurate determination of coverage. Record your tree's
identification number on the chart. Make sure your string around
the tree is 1.5 meters above the ground at all points.
Determine North, South, East, and West using a compass and mark
these points on the tree. Use the 100-circle grid at the end of this procedure* and copy it on to an acetate sheet. Place the transparent grid so that it's lower edge touches the string. The center of the grid should be lined-up with magnetic north (determined from a compass).
To observe and record percentage cover by each type of
lichen, moss, bare bark, count what is showing through each
of the 100 small circles on the acetate sheet and record the
results on the chart below. The procedure should be repeated
for each compass direction, i.e., north, south, east, west.
Each column should add up to 100.
| Tree Number________ |
North |
South |
East |
West |
| Crustose |
|
|
|
|
| Foliose |
|
|
|
|
| Fructicose |
|
|
|
|
| Moss |
|
|
|
|
| Bare Bark |
|
|
|
|
| Other |
|
|
|
|
Repeat this procedure for all 10 trees. To submit your data, average the data from the four cardinal directions.
For example; Crustose North 26) Crustose East (28), Crustose South (26), Crustose West (28) would be an
average of 27. In other words, the average crustose coverage on the tree was 27%. The number on the data
entry form would be 27.
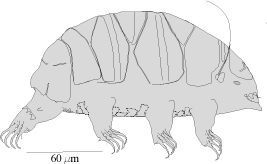
Tardigrade Sampling
Students collect samples of lichens from the bark of area trees. The samples are taken to the classroom and submerged in water to reanimate the tardigrades living in the lichens. The exercise provides the opportunity to make basic ecological calculations and introduces the concept of diversity.
|
The following procedure will be helpful in collecting lichens for use in tardigrade sampling. Lichens are sensitive slow growing organisms. Collection should be limited to necessary samples.
|
| |
|
Sampling Procedure for Tardigrades
- The lichens should be collected from the same 10 trees used for the lichen coverage study, but not
from the same area on the tree that transect data was collected. Choose some other area on the
tree.
- Use an area of bark that is 100% covered by lichens. Use a large borer, at least 1 3/32 inch, or
larger, to collect the lichens. Some samples will be easily collected while others will require that the
bark of the tree be collected also. Record the tree identification number on the data sheet.
- When students return to the classroom they should place the lichen samples in Petri dishes
(lichen down), one quarter full with filtered spring water, deionized water, or pH 7.0 distilled water.
Record the tree identification number on the Petri dish. Use a spray bottle of filtered spring water,
deionized, or with distilled water to keep the lichen moist at all times. Each lichen
sample should have it's own Petri dish. The water rehydrates desiccated tardigrades and other
invertebrates that students will be able to observe. The soaking will reanimate these animals in 2-3 hours,
but 24 hours will yield good results. Do not wait longer then 48 hours to check for the tardigardes. They will
begin to disappear from your sample. A Quick Time video
introducing Tardigrades and
basic sampling techniques is online. This is a large file. Anticipate a long download time.
- Remove the lichens and, using forceps, shake them gently upside down in the water of the petri dish.
Search the petri dishes for tardigrades. The will be on the bottom of the dish. The search should be
systematic and uniform, following a simple pattern throughout the dish. Have the students use data
table below to record numbers of tardigrades family in each sample.
- In order to compare data with other sites and make the density and diversity data meaningful it is
necessary to identify and count the tardigrades by family. To assist in determining the family of each
tardigrade, an Annotated Taxonomic Key to the Families of Terrestrial and Freshwater Tardigrades. The Dichotomous Key has been developed from the works of Ramazzotti & Maucci;1983; Schuster et al., 1980; Nelson, 1991; and Kinchin, 1994. It is intended for use to identify the common tardigrades found during the lichen survey. It does not dwell on the rare and infrequent groups. To use this Key it is necessary to make a choice between two characteristics, generally the presence or absence of a feature or structure but some times a magnitude of size must be judged. To this end we have provided drawings that concentrate on the feature or structure in question to aid in your decision making. If the students wish to use a more advanced method of classification a good reference is : Morgan, C.I., & P.E. King (1976) British Tardigrades: Keys and notes for the identification of the species. New York: Academic Press. Counts should be made three times and recorded by family on the chart below.
- Calculate the average of each type of tardigrade and
the total of each count.
|
|
Count 1 |
Count 2 |
Count 3 |
Average |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Calculating Tardigrade Density
To calculate the density of tardigrades students should
determine the lichen surface area for the sample they are observing. If
a round borer was used to collect the lichens, the area of the lichen sample
is:
Lichen Area = (3.14)(radius of the sample) 2
Density of the tardigrades is calculated by dividing the number of tardigrades observed by the area of the lichen sample. Density is calculated for each family of tardigrade by dividing the number of each family of tardigrade observed by the total surface area of the lichen.
Tardigrade Density Echiniscidae = Number of tardigrades Echiniscidae/Lichen Area
Tardigrade Density Oreellidae = Number of tardigrades Oreellidae/Lichen Area
Calculate the mean density by summing the densities of all the types of tardigrades and dividing by the number of families found.
Mean Density = (Density of Echiniscidae + Density of Oreellidae)/ Number of families found
Calculating diversity using the Simpson Diversity Index
Calculate the proportion of the total number of tardigrades
of each type (Pi).
Proportion Echiniscidae (Pa) = number of Echiniscidae/Total number
of tardigrades
Proportion Oreellidae(Pb) = number of Oreellidae/Total number of tardigrades
To calculate the Simpson Diversity Index = 1-Sum of [Pi2]
For example, if you looked at 50 tardigrades classified
as Echiniscidae and 20 tardigrades of Oreellidae you would calculate:
Diversity Index = 1- ((50/70)2) + (20/70)2)
Diversity Index = .41
This index ranges from zero to one and is literally a measure of the probability that two tardigrades taken at random from the sample are different species. A number close to zero means low diversity and it is likely you will get the same species of tardigrade and a number close to one means high diversity and you are likely to get different species from a sample.
*Directions for Creating the Transparent Grid Used in
Sampling Lichen Coverage*
The grid is composed of 100 small (1/8") circles
arranged in a 10 by 10 grid. The circles should be placed within the dimensions
of a 6 inch by 6 inch square. An online example to print is available. Beware!! printers may vary!!
|

