
Creating
the Context
Home
Research Focus
Background Info
Research Methodology
Data Submission
Results of Study
Data Analysis
Conclusion
Further Research
Guided
Research
Research Question
Background Info
Research Methodology
Data Submission
Results of Study
Data Analysis
Conclusion
Further Research
Research Values
Student
Research
Doing Research
Publish
View
|
About Tardigrades |
Tardigrade Studies | Tardigrade Multimedia
|
TARDIGRADES: BEARS OF THE MOSS (About Tardigrades)
by William R. Miller, Ph.D.
Originally published in The Kansas School Naturalist, May
1997.
The images below can be enlarged by clicking on them.
|
|
"Tarde what?", is a question I am often asked, generally elicited by the pictures and drawings like the one on the right.
With that setup, I can explain an animal you cannot see, have never
heard of, and that causes no harm.
|
| |
I get to convey the adventure of discovery, the excitement of finding
animals that few humans have ever seen. You fell into my trap when you picked up this booklet, so take a few
minutes and maybe you too will be intrigued by tardigrades, Bears of the Moss.
Tardigrada is one of several little-known phyla of invertebrates located between the nematodes
(roundworms) and the arthropods (crustacea, insects, ticks and mites). They are small, 0.2-0.5 mm in length,
about the size of a dot made with a "fine" mechanical pencil. A big tardigrade can be seen with the naked eye,
but the light must be just right.
|
|
|
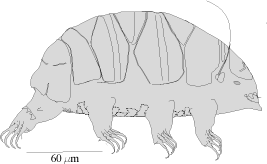
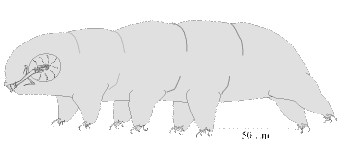
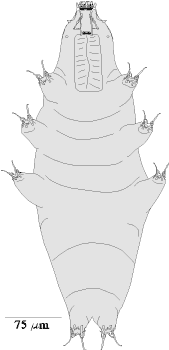
Tardigrades look like miniature caterpillars with five body segments and four pairs of clawed legs
(Figure 1&2). Like "higher" animals they have digestive, excretory, and nervous systems; separate sexes;
and well-developed muscles. Like "lower" animals, they lack respiratory and circulatory systems, instead, they
breathe through their skin or cuticle and the whole body acts as a pump to circulate fluids.
|
The word tardigrade means "slow walker" which describes their rather sluggish, clumsy movement. They
use their short legs and claws to cling to a substrate and waddle along like "Water Bears" or "Moss Piglets"
(Kinchin 1994). Tardigrades are very common and have been found on every continent (McInnes 1994). They have
been recorded in every biotope: both salt and fresh water; the humidity of rain forests, the altitude of
mountains, the dryness of desserts, and the isolation of remote islands and Antarctic nunatacks.
All tardigrades are aquatic. They need to be in water to live, to find food, to breathe, to reproduce,
and to move. There are marine, freshwater, and limno-terrestrial species. The latter are the subject of this
booklet and live in the water droplets trapped in the space between the leaves of moss cushions, the thalli of
lichens, and leaf litter. Here they share a micro-world with other organisms (collembola, mites, rotifers, and
nematodes) and endure extreme environmental cycles from flood to drought.
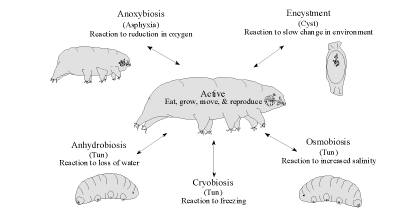
The reason tardigrades are "interesting" is that they have developed ways to survive these
environmental swings. They survive times of flood or lack of oxygen by swelling up like a balloon
(anoxybiosis) and floating around for a few days. They survive until the environment dries, then return to the
active state for feeding, growing, and reproducing (Figure 3).
When the environment dehydrates in dry weather tardigrades desiccate into a reversible state of
metabolic suspension called cryptobiosis. They shrivel to about one-third their former size into a wrinkled
"tun" (Figure 3). Individuals have been observed to come and go from the cryptobiotic state repeatedly and
tardigrades have been reported to survive more than 100 years (Kinchin 1994).
Cryptobiosis is of great interest in the study of cryogenics and tardigrades have been subjected to
laboratory experiments which verified their ability to survive. Tardigrades have tolerated temperatures below
freezing at 0.05K (-272.95 C) for 20 hours and -200 C for 20 months. They have survived 120 C, pressures of
1000 atmospheres, and high vacuums. In the cryptobiotic state, tardigrades have shown resistance to hydrogen
sulfide, carbon dioxide, ultraviolet light, and X-rays (Kinchin 1994). We could speculate that tardigrades
could be transported through outer space in their existing form.
Despite these "capabilities", tardigrades are still little understood. In the 200 years since the
waterbear was first described, we have not identified any specific medical, commercial, or environmental
effect of tardigrades. We have identified three Classes, five Orders, 15 Families, 94 genera and more than 750
species (Table 1).
|
|
COLLECTING TARDIGRADES
Tardigrades are easy to collect. No special equipment is needed; most is available at the grocery
store. Limno-terrestrial tardigrades can be found in moss cushions growing on rocks, soil, or the side of
houses. Lichens that grow on the trunks of trees and rocks support a good mix of tardigrade species.
|
|
|
To collect moss, simply pull off a clump of the cushion with your fingers and place it in a small paper
bag. School lunch bags work best. Do not use plastic bags since the moist moss will not dry and allow the
tardigrades to become cryptobiotic. A pocket knife can be used to scrape lichens into a bag. For precise,
comparative samples, use a core sampler, to collect equal areas. A cubic sample of moss can be analyzed for an
internal habitat (Miller, Miller & Heatwole 1994).
Collect only a sufficient quantity for plant and animal identifications. Do as little environmental
damage as possible. Write your collection code on the paper bag and on a map to mark the location. Enter the
collection code in your field notebook along with the description of the location, plant type, substrate,
conditions, and other relative data such as exposure, sea spray, or road dust.
WORKING WITH TARDIGRADES
Place part of the moss or lichen sample in bowls or cups and add 100 ml of distilled water. Plastic
picnic cups work well. However, tap water containing chlorine may prevent your tardigrades from emerging from
cryptobiosis. Keep the remainder of the sample for plant identification.
To observe active tardigrades after only an hour or two of soaking, pipette a small quantity of the
debris from the bottom of the bowl into a petri dish. Place the dish under a dissecting microscope at 40X.
Normal transmitted light will work but reflected light directed from the side of the dish at a 45 angle
produces a clearer image. Use a black background under the dish.
Tease the debris apart with a fine needle or insect pin taped to a pencil. Tardigrades will be seen
wiggling on the bottom of the dish or crawling along a piece of debris. The first one is the hardest to see;
the rest will be easy. For the best view, put a drop of water on a microscope slide, insert a tardigrade and
drop on a coverslip. The action is spectacular, both the internal and external features can be seen, you will
fall in love with tardigrades at this point.
Eggs are very important for identification of some species, so spending the time to look for them is
necessary. They will appear as small white or bright sparkles, little Christmas tree ornaments or sea mines.
Search under 40-80 power. This may be hard on the eyes but possible with practice.
|
|
To accumulate specimens for study, let the sample stand in the water for 24to 36 hours. Then squeeze
the plant material with your hand or fingers to dislodge any remaining animals.
|
|
The asphyxiated (anoxybotic)
specimens can be separated and moved with a micro-pipette or a very fine inoculation loop (Irwin loop).
Specimens should be preserved in small viles of Ethel alcohol at a 70% concentration or buffered formalin at a
5% solution. Insert a label with the collection code into the vile and mark the outside with a permanent
marker.
|
|
To make slides, tardigrades are generally mounted in Hoyer's medium (Table 2) which acts both as a
mounting medium and a clearing agent. Preserved or asphyxiated tardigrades may be mounted. Center a drop of
Hoyer's on glass slide and transfer specimens to the medium. Gently place a glass coverslip onto the drop.
Close attention should be paid to the final location of the specimen as the cover slip will cause smaller
specimens to flow with the medium as it evens out. To reduce the searching time later, use a fine pointed felt
tip pen and place a dot on the coverslip to mark the location of the animal. Eggs are mounted in Hoyer's
medium the same as the animals but because of their small size (0.050-0.100 mm) are more challenging to handle.
Slides should be immediately labeled with the collection code and put aside to dry for a couple of
weeks. After drying, seal the coverslip by applying a coat of epoxy paint to the edge with a fine brush.
Slides must be stored flat because the Hoyers never completely dries and the specimens will move if stored on
their edge. Slides may be examined under medium power in a day or two. Slides should be dry and sealed before
observing under oil emersion high power magnifications.
Most tardigrades can be identified to genus at the medium powers (200-400X). High power (1000X) is
generally required for determining species. Tardigrades are generally drawn with a camera lucida attachment.
Photography can be accomplished with camera attachments. A video image may be viewed on a television. Video
may also be digitized on a personal computer, printed on a laser printer, and stored on a disk. Most of the
drawings in this booklet are made from digitized video images, a technique that preserves dimensions and
relationships of structures.
|
|
STUDYING TARDIGRADES
In your collection of moss or lichens, two basic types of limno-terrestrial tardigrades will be found.
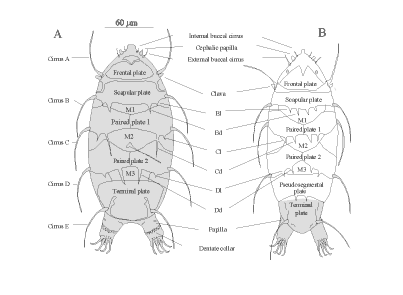
Armored specimens are in the Class Heterotardigrade, Order Echiniscoidae (Figure 1; Table 1). Naked specimens
are in the Class Eutardigrada, Order Parachela or Apochela (Figure 2; Table 1).
|
|
|
The armored Heterotardigrades will have spotted, pitted, or sculptured dorsal and lateral plates of
varying number, size, and shape. Many are red in color. They may have spines of varying length and thickness
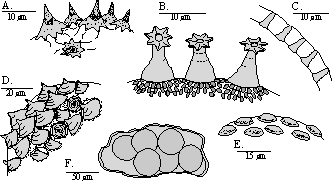
projecting from the plates. Heterotardigrades have four separate claws on each foot (Figure 8B). The
mouthparts (Figure 7B) are often obscured by the cuticular plates. Variations of these features determine genus
and species.
|
|
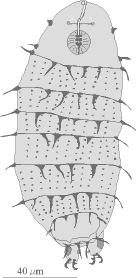
The naked Eutardigrades do not have the dorsal plates but a few may exhibit some reticulations,
sculpturing, bumps, or even spines (Figures 10, 11). They have a pair of branched claws that vary in size and
shape on each leg (Figure 8C-I). Detail of the buccal apparatus is generally visible (Figures 7C-I). Different
genera and species may have placoids, teeth, and lamella of varying number and size and exhibit variation in
other structures that can be used for identification.
|
WHAT YOU ARE LIKELY TO FIND
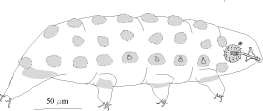
Members of the genus Echiniscus are cosmopolitan, moderate sized (0.10-0.35 mm in length), and armored
Heterotardigrades. They are identified by the number, shape, and placement of their dorsal armored plates and
spines or cirrus. A dentate collar on the fourth pair of legs has teeth and the internal claws may have spurs,
the external claws are smooth (Figure 8B). The eggs are oval and deposited in the exuvium.
|
|
|
Two general types of Echiniscus are recognized, the first has only one lateral or dorsal spine, the
most anterior cirrus "A." The granulation on their plates is dense and uniform. The internal claws have large
curved spurs and the dentate collar on fourth pair of legs has many sharp teeth (Figures 8B). The other type
is distinguished by having several lateral and/or dorsal spines present on the edges of any or all of the
dorsal plates (Figure 14). They generally have red eyes, double granulation composed of a very fine, uniform
sculpture, and a courser set of depressions or pores of irregular size and arrangement. The dentate collar has
small irregular teeth.
Pseudechiniscus are small (0.10-0.20 mm) red, armored Heterotardigrades with a pseudo-segmental plate
inserted between median plate three and the terminal plate. They have large, black eyes and very fine
granulation. Only lateral appendages are a cirrus and clavae arising between the head and first plate, other
appendages are absent (Figures 1). Medial plates I and II are divided; median plate III is single. The
internal claws on all legs have a spur just above base. A dentate collar is not present on the fourth pair of
legs.
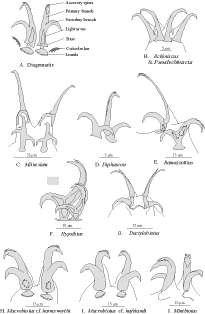
Milnesium is a genus of large (up to1.0 mm in length), very common, cosmopolitan tardigrades.
Milnesium is distinguished by its anteriorly sloping body, the very wide buccal tube, the muscular pharynx
without placoids. Six oral papillae and triangular lamellae surround the mouth opening (Figure 7C). The claws
are distinctive with a long, thin primary branch and a short, multi hooked secondary branch (Figure 8C). It is
a carnivorous species and is occasionally observed eating nematodes. The mouth parts of rotifers and the
buccal apparatus of other tardigrades may occasionally be observed in its gut.
|
|
Diphascon is a genus of medium-sized (0.20-0.40 mm), smooth, eyed tardigrades with long, thin bodies.
The buccal tube is also very thin. The esophageal tube is very long, thin and leads to an elongated pharynx.
Long, thin macroplacoids are present. A microplacoid and a septulum may be present (Figure 7D). Macroplacoids
usually increase in size from the first to the last. Cuticular bars are often present at the base of
hypsibius-type claws.
The Ramazzottius genus contains common cosmopolitan mid-sized (0.20-0.35 mm in length) tardigrades with
a granular cuticle that has a pigmented (red or brown) pattern often appearing in longitudinal rows and
transverse bands. The buccal tube is narrow, the pharynx oval to round (Figure 7E). An apophysis is present
as are two roundish, smooth macroplacoids, nearly equal in size. The claws are oberhaeuseri-type (Figure 8E)
with the primary branch of the external claws very thin, long, and whip-like.
Hypsibius is a common genus of smooth tardigrade with or without eyes and up to 0.35 mm in length.
They have a short, broad buccal tube and a round pharynx. In the pharynx is a large apophysis, two elongated,
globular macroplacoids, and no microplacoid (Figure 7F). The claws are typical hypsibius-type in the 1-2-1-2
pattern (Figure 8F). The eggs are generally smooth and left in the cuticle, but some species do leave free eggs
with small projections (Figure 6C).
|
|
Dactylobiotus is a large (0.30-0.50 mm), smooth, aquatic genus of tardigrade. A broad buccal tube
leads to an oval pharynx with two slender macroplacoids, the first twice the length of the second (Figure 7G).
No microplacoids are present. The large, thin claws are connected at their base with a chitinous bar (Figure
8G). The eggs have small, cone shaped projections and are laid free (Figure 6D).
|
|
|
The Macrobiotus cf. harmsworthi group contains medium to large (0.20-0.50 mm), cosmopolitan tardigrades
with a smooth cuticle and eye spots. The supported buccal tube is wide. The oval pharynx has three
macroplacoids and a large microplacoid (Figure 7H). The claws are Y-shaped with small lunules. Eggs have
prominent projections that look like cones rising from the surface (Figure 6A). Eggs are required for
identification.
Members of the Macrobiotus cf. hufelandi group are large (0.30-0.50 mm), white tardigrades with a
smooth cuticle and eye spots. The supported buccal tube is moderate in width. Two macroplacoids and a
microplacoid are found in the elongated pharyngeal bulb. The first macroplacoid is longer than the second and
often constricted to appear as two (Figure 7I). They have Y-shaped claws with prominent lunules and accessory
points (Figure 8I). The eggs are deposited free, with ornamentation that resembles inverted egg-cups or goblets
(Figure 6B). Many species in this group are very difficult to separate even for experts. Eggs are required.
|
|
|
The Minibiotus genus is composed of small (0.10-0.25 mm), smooth tardigrades with large eye spots.
They have three round equal sized macroplacoids in a round pharyngeal bulb and a tiny microplacoid (Figure 7J).
The stylet supports are joined to the buccal tube at the midpoint of its length. The claws are small of the
hufelandi type with large accessory spines and large lunules (Figure 8J). The eggs have smooth dome-like
projections (Figure 6E).
TARDIGRADE LIFE
Naked Eutardigrades hatch as small versions of the adults and grow by molting through instars that are
similar but larger. The armored Heterotardigrades change from one molt to the next, achieving their
distinctive morphology as adults. Changes include the number and size of dorsal spines, texture of plates, and
the appearance of the gonopore.
The active life of a tardigrade may last only a few months, although it may be spread over several
years if interrupted by cryptobiotic periods. Growth and molting occur throughout the active life and from six
to 12 instars are common (Higgins 1959). Molting begins with the 'simplex' stage where the feeding apparatus
is lost. The old cuticle is shed and left behind. Then a new feeding apparatus is regenerated. Tardigrades
do not feed during molting which may take hours to a few days.
Tardigrades exhibit both sexual and asexual reproduction. Parthenogenetic strains are known where
females produce females, without fertilization. For bisexual limno-terrestrial tardigrades, periods of
reproduction occur quickly because of the erratic nature of cryptobiosis. Many tardigrades leave their eggs in
their discarded skin when they molt. Others release their eggs free into the environment. Egg producing
activity generally declines in the winter.
The egg may be smooth, have rounded projections, thin spines, or robust cones. Some projections are
split or branched and the surface of the egg may be smooth, pitted, or patterned (Figures 4, 7). The size,
shape, and pattern of the projections and the surface of the egg are the only known differences between some
species of tardigrade, thus eggs are needed for identification.
The buccal apparatus of tardigrades show great variation in detail but great similarity in structure
and function. Generally the mouth and buccal tube are flanked by two piercing stylets. A pharyngeal tube leads
to the bulbus, sucking pharynx that opens into the alimentary system (Figures 7). Mouthparts are used in
identification. The claws also show great diversity from simple, smooth sword-like to multi branched tree-like
structures (Figures 8). They are heavily used in taxonomic determination.
|
| |
1999, KanCRN Collaborative Research Network | |

